Search
Sharing Knowledge improves Knowledge... Knowledge should come at as less cost as possible.
Posts
Showing posts from December, 2013

Posted by
Varun C N
Fighting tuberculosis
- Get link
- Other Apps

Posted by
Varun C N
Bacteria count each other using homoserine lactones or peptides- Quorum sensing
- Get link
- Other Apps
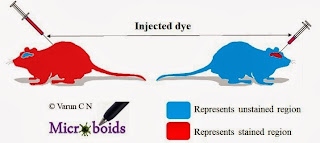
Posted by
Varun C N
Isolated Immunology inside Central Nervous system
- Get link
- Other Apps