Search
Sharing Knowledge improves Knowledge... Knowledge should come at as less cost as possible.
Posts
Showing posts from August, 2016

Posted by
Varun C N
The Rise of Superbug Gonococcus
- Get link
- Other Apps

Posted by
Varun C N
Indigenously developed Leprosy vaccine
- Get link
- Other Apps
Posted by
Varun C N
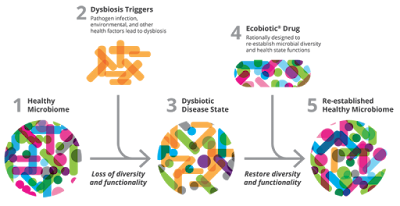
SER109- Phase 2 trial doesn't look good
- Get link
- Other Apps

Posted by
Varun C N
Zika infection- Updates II
- Get link
- Other Apps

Posted by
Varun C N
S Typhimurium Persistence model
- Get link
- Other Apps