Search
Sharing Knowledge improves Knowledge... Knowledge should come at as less cost as possible.
Posts
Showing posts from November, 2012

Posted by
Varun C N
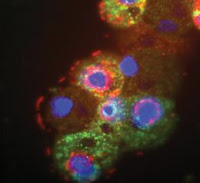
A Rival for HIV
- Get link
- Other Apps
Posted by
Varun C N
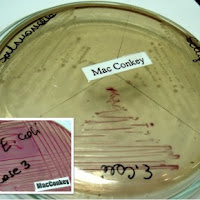
Virophage, Viroporins and Trogocytosis
- Get link
- Other Apps
Posted by
Varun C N
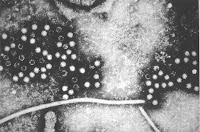
Dynamic Gut Microbiome
- Get link
- Other Apps

Posted by
Varun C N
The puzzling Vomocytosis
- Get link
- Other Apps
Posted by
Varun C N
New interest in Hecolin
- Get link
- Other Apps