Avibactam- Non β-lactam/β-lactamase inhibitor
Greetings,
Avibactam has a broad spectrum of activity against classes A, C and some members of class B serine -β lactamases. The mechainsm of action is to form a covalent bond with β-lactamases that is slowly reversible, reforming the avibactam molecule and the β-lactamase enzyme. This mechanism can help in reducing MICs, upto 1024 times reduction (Massive activity) which restores susceptibility to several existing β lactam drugs. The plus points include it can be used in combination with ceftaroline against selected β-lactamase- producing anaerobic strains such as Bacteroides fragilis, Prevotella species, Finegoldia magna, Enterobacter spp. Morganella etc. It also has excellent activity in combination with ceftaroline (antistaphylococcal cephalosporin). It is also useful in treating P. aeruginosa mutants producing the class A PER-1 ESBL. However, they are not active against OXA ESBLs or the VEB-1 enzyme producing P. aeruginosa strains and relatively ineffective against carbapenem- resistant A. baumannii.
Based on studies a confident comment has been made by Marco Taglietti (18 October 2011), “We are pleased to move forward with the CAZ-AVI development programme. This combination of a broad-spectrum cephalosporin and a novel beta-lactamase inhibitor has the potential to be effective against bacteria that would otherwise be resistant to antibiotics in patients suffering from serious and potentially life-threatening infections.”. Reference The drug is currently in Phase III clinical trials, developed through Generating Antibiotic Incentives Now (GAIN) Act, which was part of the FDA Safety and Innovation Act (FDASIA), a fast track development method.
In my previous post, I emphasized the fact that the Clinical side of Microbiology are in a real need of Antibiotics. A recent review article published in nature by Butler etal, focusses on recent antibiotic under development. The review discusses new antibiotics launched since 2000, including the most recent addition fidaxomicin and bedaquiline. During my reading, I stumbled on a beta-lactamase inhibitor called as avibactam. Thought this is a good time for me to post on β-lactams and β-lactamase inhibitor.
Resistance to β-Lactam antibiotic is one of the most documented and well studied modes of drug resistance. This may be attributed to the most widespread use of β- lactam antibiotics. β-lactamases are enzymes (EC 3.5.2.6) produced by some bacteria and are responsible for their resistance to beta-lactam antibiotics like penicillins, cephalosporins (are relatively resistant to β- lactamase), cephamycins, and carbapenems. They are Serine proteases that belong to the same family as that of the β-lactam antibiotic and PBP (high and low molecular weight types). Currently more than 300 possible varieties of β-lactamases are known that can produce significant clinical resistance. The classification of β-lactamase is based on two systems of classification- Molecular and Functional classification.
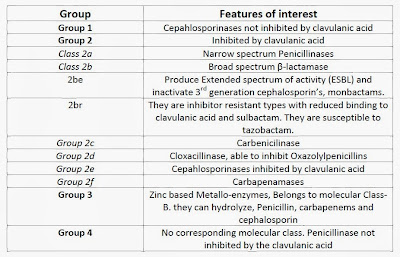 |
| Table 1: Functional classification of β-lactamase. |
Molecular classification:
The molecular classification of β-lactamases is based on the nucleotide and amino acid sequences of the enzymes. To date, four classes are recognized (A-D), correlating with the functional classification. Classes A, C, and D act by a serine-based mechanism, whereas class B or metallo-β-lactamases need zinc for their action.
Functional classification:
The functional classification is based on the spectrum of activity of the β-lactamase enzyme. The classification is extensive and is useful clinically. It is more widely accepted and followed in most of the countries. According to this classification β-lactamase is divided into 4 main groups (Group 1-4) based on type of action and further sub classified based on the spectrum of activity.
As a mechanism to combat the ever rising number of beta lactamases, β-lactamase inhibitors were brought into clinical picture. A β-lactamase inhibitor has a higher affinity to bind the β-lactamase enzyme and thus spares the active β-lactam antibiotic. The drug is commonly given as BL/BLI ( β-lactam/β-lactamase inhibitor) combination. One of the best said example is Augmentin. Other BLI's in common use include Sulbacatm and Tazobactam.
Please note, in all the above said BL/BLI combination, the BLI is a dummy β-lactam drug, without activity in itself. The next generation of BLI are Avibactam and MK-7655. Unlike their ancestors, these are diazabicyclooctane (DABCO) inhibitors and thus not β-lactams themselves. Hence they are also referred to as the (Non β-lactam/β-lactamase inhibitor) NBL/BLI inhibitor.
I quote the following from nature article (Original paper, Link)
"Avibactam was discovered by Hoechst Marion Roussel, which eventually formed part of Sanofi-Aventis (Paris, France). Sanofi-Aventis spun out anti-infective discovery into Novexel in 2004, which was acquired by AstraZeneca (London, UK) in 2010. Avibactam is also being evaluated in phase-II and phase-I trials in combination with ceftaroline and aztreonam, respectively".
Please note, in all the above said BL/BLI combination, the BLI is a dummy β-lactam drug, without activity in itself. The next generation of BLI are Avibactam and MK-7655. Unlike their ancestors, these are diazabicyclooctane (DABCO) inhibitors and thus not β-lactams themselves. Hence they are also referred to as the (Non β-lactam/β-lactamase inhibitor) NBL/BLI inhibitor.
I quote the following from nature article (Original paper, Link)
"Avibactam was discovered by Hoechst Marion Roussel, which eventually formed part of Sanofi-Aventis (Paris, France). Sanofi-Aventis spun out anti-infective discovery into Novexel in 2004, which was acquired by AstraZeneca (London, UK) in 2010. Avibactam is also being evaluated in phase-II and phase-I trials in combination with ceftaroline and aztreonam, respectively".
 |
| Fig 1: Avibactam. Source |
Based on studies a confident comment has been made by Marco Taglietti (18 October 2011), “We are pleased to move forward with the CAZ-AVI development programme. This combination of a broad-spectrum cephalosporin and a novel beta-lactamase inhibitor has the potential to be effective against bacteria that would otherwise be resistant to antibiotics in patients suffering from serious and potentially life-threatening infections.”. Reference The drug is currently in Phase III clinical trials, developed through Generating Antibiotic Incentives Now (GAIN) Act, which was part of the FDA Safety and Innovation Act (FDASIA), a fast track development method.
Butler MS, Blaskovich MA, & Cooper MA (2013). Antibiotics in the clinical pipeline in 2013. The Journal of antibiotics, 66 (10), 571-91 PMID: 24002361
Shlaes DM (2013). New β-lactam-β-lactamase inhibitor combinations in clinical development. Annals of the New York Academy of Sciences, 1277, 105-14 PMID: 23346860
Goldstein EJ, Citron DM, Merriam CV, & Tyrrell KL (2013). Comparative in vitro activity of ceftaroline, ceftaroline-avibactam, and other antimicrobial agents against aerobic and anaerobic bacteria cultured from infected diabetic foot wounds. Diagnostic microbiology and infectious disease, 76 (3), 347-51 PMID: 23623385





I have been through understanding BLI but you really clarified through the mode of action of Avibactam. Looking forward for more interesting posts.
ReplyDelete