Search
Sharing Knowledge improves Knowledge... Knowledge should come at as less cost as possible.
Posts
Showing posts from August, 2013

Posted by
Varun C N
HIV latency @ a glance
- Get link
- X
- Other Apps

Posted by
Varun C N
New vaccine candidate against Dengue virus- MTase mutants
- Get link
- X
- Other Apps

Posted by
Varun C N
Rhabdovirus-derived particles against leukemia
- Get link
- X
- Other Apps

Posted by
Varun C N
Attenuated Sporozoites in Blood is Protective?
- Get link
- X
- Other Apps

Posted by
Varun C N
Viral DNA? Lets do a STING operation
- Get link
- X
- Other Apps

Posted by
Varun C N
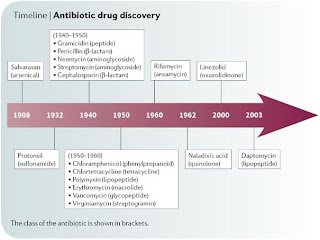
Attacking ATP synthase- Strategy against TB
- Get link
- X
- Other Apps
Posted by
Varun C N
Addictive Bacterial control system- TAT
- Get link
- X
- Other Apps