Search
Sharing Knowledge improves Knowledge... Knowledge should come at as less cost as possible.
Posts
Showing posts from November, 2013
Posted by
Varun C N
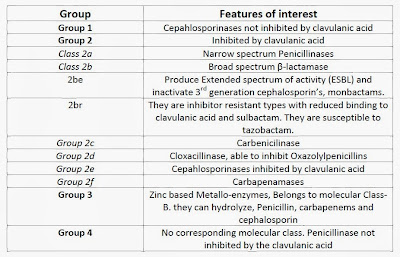
Avibactam- Non β-lactam/β-lactamase inhibitor
- Get link
- Other Apps

Posted by
Varun C N
Acyldepsipeptides (ADEPs) active against biofilm producers
- Get link
- Other Apps

Posted by
Varun C N
Retroviral genome remnants influence Neural functions
- Get link
- Other Apps

Posted by
Varun C N
Debunking Credited Myths
- Get link
- Other Apps

Posted by
Varun C N
Viral Fusion Proteins
- Get link
- Other Apps