Superdrug for the Superbug
Antibiotic resistance is a huge problem and the problem of so-called "Superbugs" is not new to the readers. There are several reports of resistance against drugs that are considered as reserved only as last resort. Colistin is an example, which is preferentially used against gram negatives as the last drug of choice. Similarly, Vancomycin is a reserved antibiotic for gram positives, especially MRSA. MRSA now being an absolutely common isolate vancomycin is more commonly used.
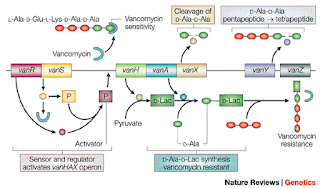 |
| Fig 1: Mechanism of Vancomycin resistance. Source |
Vancomycin (Initially code-named as 05865 and later marketed as Vancocin) was first isolated by Edmund Kornfeld in 1953 from a soil sample collected from Amycolatopsis orientalis from the interior jungles of Borneo. The toxicity was a limiting factor and antibiotic was reserved only for those cases, where nothing else worked. Chemically, Vancomycin is a glycopeptide (branched tricyclic glycosylated nonribosomal peptide) and acts by inhibiting cell wall synthesis. Vancomycin binds to the terminal D-Ala-D-Ala dipeptide which halts the process of peptidoglycan formation. The resistance is due to change in D-Ala-D-Ala. The most common change is D-Ala-D-Lac which has orders of low binding of vancomycin. See Fig 1, for details.
Vancomycin resistance has been a huge problem in health care settings. There are several genes that mediate resistance to vancomycin named as VanA, VanB, VanC, VanD and VanE. The commonly talked about vancomycin resistant strains are VRSA (Vancomycin resistant Staphylococcus aureus) and VRE (vancomycin resistant Enterococcus species). These are very difficult to treat infections. The most common mechanism of glycopeptide resistance is to develop modified cell membrane receptors with reduced affinity. The problem has been recognised quite early and one of the obvious methods of dealing with it is to modify the vancomycin. As early as 1999, a collaborative group between Princeton and Merck showed that the carbohydrate derivatives of vancomycin were able to overcome resistance to vancomycin.
 |
Fig 2: Structure of[Ψ[C(═NH)NH]Tpg4]vancomycin
aglycon. Source
|
There are several reports in the literature reporting successful modifications of vancomycin increasing its kill capabilities. Many of these were loosely called as Vancomycin ver 2.0. In 2011, Dale Bogger's lab from Scripps Institute reported the development of a new vancomycin derivative a [Ψ[C(═NH)NH]Tpg4] vancomycin aglycon which could bind both d-Ala-d-Ala and d-Ala-d-Lac.
 |
| Fig 3: Structure of modified vancomyin 3.0. Source |
Improving on their work Bogger's lab has now reported the design of a Vancomycin 3.0 (CBP C1- aminomethylene vancomycin). This chemical has 3 different modes of activity. Not only it has a dual binding activity, the addition of quaternary ammonium salt in the structure (It was a calculated design), provided a binding pocket-modified vancomycin analogue which was independent of D-Ala-D-Ala or D-Ala-D-Lac binding. Another modification of peripheral (4-chlorobiphenyl) methyl (CBP) to the vancomycin disaccharide made it highly potent. The same drug also can induce cell permeability. Basically, the same drug has 3 independent mechanisms of action, making it difficult for the bacteria to acquire resistance. Indeed, there is data to show that in vitro the bacteria didn't acquire any resistance even after 50 passages.
As Bogger States, "Organisms just can't simultaneously work to find a way around three independent mechanisms of action. Even if they found a solution to one of those, the organisms would still be killed by the other two".
The compound is not yet ready for human clinical use. The chemical synthesis is very laborious and it is not known as to what are its side effects.
References:
Levine D. Vancomycin: A History. Clinical Infectious Diseases. 2006;42(Supplement 1): S5-S12.
Ge M. Vancomycin Derivatives That Inhibit Peptidoglycan Biosynthesis Without Binding D-Ala-D-Ala. Science. 1999;284(5413):507-511.
Xie J, Pierce J, James R, Okano A, Boger D. A Redesigned Vancomycin Engineered for Dual d-Ala-d-Ala and d-Ala-d-Lac Binding Exhibits Potent Antimicrobial Activity Against Vancomycin-Resistant Bacteria. Journal of the American Chemical Society. 2011;133(35):13946-13949.
Okano A, Isley N, Boger D. Peripheral modifications of [Ψ[CH 2 NH]Tpg 4 ]vancomycin with added synergistic mechanisms of action provide durable and potent antibiotics. PNAS. 2017;:201704125.





Comments
Post a Comment