Hydrophobins- A snapshot
Hello fellows
Since the inception of this blog i have written about antibiotics, bacteria, viruses and some elementary frequently asked questions in microbiology and more. But, i have candled very little on fungus. So this time let me blog on a mycology topic- "Hydrophobins". Ah and let me mention, the push to write on this comes from an article published in PloS pathogens titled "Hydrophobins- unique fungal proteins".
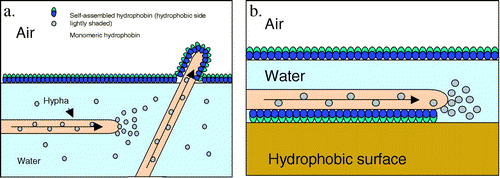
"Hydrophobins are surface active proteins produced by filamentous fungi". The Hydrophobins are a group (I better call them a family) of proteins secreted by the fungus usually less than 20kDa (with aprox 100 amino acids in it) in size, characterized by high hydrophobic nature and 8 conserved cysteine residues. They are implicated in many functions, one of them being involvement in the formation of aerial structures and in the attachment of hyphae to hydrophobic surfaces.
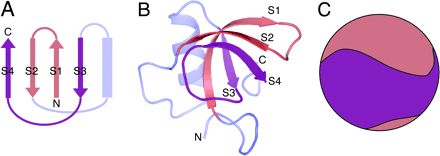
Fig 1: Topology and structure of HFBII (Click here for source)
Fig 2: Hydrophobins in formation of aerial structures (Han A. B. Wösten)
The entire class of hydrophobin is divided into 2 subgroups- Class I and Class II. The Class I hydrophobin aggregates are relatively insoluble, and can be dissociated by reagents such as formic acid or trifluoroacetic acid. The class II in contrast, aggregates are more easily soluble in 60% ethanol and 2% SDS. Why? Because, In class I, there is a considerable variation in the inter-Cys-spacing; these hydrophobins assemble into highly insoluble polymeric monolayers composed of fibrillar structures known as rodlets and difficult to shake of. On the other hand the inter-Cys spacing are more conserved in class II.
Class I: X25-158-C-X5-9 -C-C-X4-44-C- X7-23 -C-X5-7-C-C-X6-18 -C-X2-13
Class II: X17-165-C-X7-10-C-C- X11 -C-X15/16-C-X6-9-C-C-X10/11-C-X3-8
Amino acid alignment in hydrophobins (Click here for source)
(Length of the amino acid sequences between eight cysteine residues in class I and class II hydrophobins. X represents any other amino acid than cysteine and the sub-index the number of amino acids.)
The hydrophobins are known to be one of the strongest of the surfactants known with some strange biophysical properties. The atomic structure HFBII was determined by Johanna Hakanpaa etal. The structure uncovers that the protein is a single domain structure with one α-helix and four anti-parallel β-strands that completely envelop two disulfide bridges. It is calculated that HFBII is able to reduce the surface tension of water from 72 mJ/m2 to 28 mJ/m2 at a concentration of 20 μg/ml (That's incredible; for details on surface tension click here). In case of class I such as EAS, the structure is monomeric but mostly unstructured in solution, except for a small region of anti-parallel beta sheet that is probably stabilized by four intramolecular disulfide bonds (Link).
Importance of Hydrophobins:
The importance of these molecules in the field of medicine, is just emerging. The Hydrophobins are thought to be an important factor in virulence. This is at least an hypothesis in case of A. fumigatus (Source), Beauveria bassiana (Source) and possibly many more.
In the article the authors state that, therapeutic use would include generating a hydrophobin- based nanoparticle with embedded therapeutic proteins and molecules that can be released in a well defined control fashion, without bumping an immune response . (The original sentence reads "From a therapeutic point of view, the degradation-resistance and immunologically inert properties of hydrophobins could be used to generate hydrophobin- based nanoparticles with embedded therapeutic proteins and molecules that have to be slowly released within the host or transported to a specific body location without being recognized by the host immune system.".
Fig 3: Hydrophobins (Bayry et al)

Bayry J, Aimanianda V, Guijarro JI, Sunde M, Latgé J-P (2012) Hydrophobins—Unique Fungal Proteins. PLoS Pathog 8(5): e1002700. doi:10.1371/journal. ppat.1002700
Further Reading:
1. Johanna Hakanpää etal; Atomic Resolution Structure of the HFBII Hydrophobin, a Self-assembling Amphiphile. The Journal of Biological Chemistry, January 2, 2004; 279, 534-539. (Link)
2. Zefang Wang etal. Characterization and application of hydrophobin-dispersed multi-walled carbon nanotubes. Volume 48, Issue 10, August 2010, Pages 2890–2898. (Link)
Class I: X25-158-C-X5-9 -C-C-X4-44-C- X7-23 -C-X5-7-C-C-X6-18 -C-X2-13
Class II: X17-165-C-X7-10-C-C- X11 -C-X15/16-C-X6-9-C-C-X10/11-C-X3-8
Amino acid alignment in hydrophobins (Click here for source)
(Length of the amino acid sequences between eight cysteine residues in class I and class II hydrophobins. X represents any other amino acid than cysteine and the sub-index the number of amino acids.)
The hydrophobins are known to be one of the strongest of the surfactants known with some strange biophysical properties. The atomic structure HFBII was determined by Johanna Hakanpaa etal. The structure uncovers that the protein is a single domain structure with one α-helix and four anti-parallel β-strands that completely envelop two disulfide bridges. It is calculated that HFBII is able to reduce the surface tension of water from 72 mJ/m2 to 28 mJ/m2 at a concentration of 20 μg/ml (That's incredible; for details on surface tension click here). In case of class I such as EAS, the structure is monomeric but mostly unstructured in solution, except for a small region of anti-parallel beta sheet that is probably stabilized by four intramolecular disulfide bonds (Link).
Importance of Hydrophobins:
The importance of these molecules in the field of medicine, is just emerging. The Hydrophobins are thought to be an important factor in virulence. This is at least an hypothesis in case of A. fumigatus (Source), Beauveria bassiana (Source) and possibly many more.
In the article the authors state that, therapeutic use would include generating a hydrophobin- based nanoparticle with embedded therapeutic proteins and molecules that can be released in a well defined control fashion, without bumping an immune response . (The original sentence reads "From a therapeutic point of view, the degradation-resistance and immunologically inert properties of hydrophobins could be used to generate hydrophobin- based nanoparticles with embedded therapeutic proteins and molecules that have to be slowly released within the host or transported to a specific body location without being recognized by the host immune system.".
Fig 3: Hydrophobins (Bayry et al)
Bayry J, Aimanianda V, Guijarro JI, Sunde M, Latgé J-P (2012) Hydrophobins—Unique Fungal Proteins. PLoS Pathog 8(5): e1002700. doi:10.1371/journal. ppat.1002700
Further Reading:
1. Johanna Hakanpää etal; Atomic Resolution Structure of the HFBII Hydrophobin, a Self-assembling Amphiphile. The Journal of Biological Chemistry, January 2, 2004; 279, 534-539. (Link)
2. Zefang Wang etal. Characterization and application of hydrophobin-dispersed multi-walled carbon nanotubes. Volume 48, Issue 10, August 2010, Pages 2890–2898. (Link)






Comments
Post a Comment