Speaking Phage language
Host Pathogen relationship is sometimes a horribly complicated molecular network. Merely infecting a host and continuing to do so, is not the goal of the pathogen (See my earlier post). Bacteria has been well studied in this contex. Quorum sensing is a method of establishing communication with other bacteria to determine what would be the right time to carry out a particular activity. Similar communication system exist in fungal and parasitic world, though they havent been studied in extensive detail.
Viruses are different. It is not sure if we should even call the viruses as a living entity. Bacteriophages are viruses that infect bacteria with most phages having 2 types of life cycle to choose from. Though on the overview it appears that lytic or lysogenic cycle is a random choice for phage, it is not so. It is known that the number of host available to infect has an has an influence on choice of life cycle. Think about it. If there are too few bacteria for the phage to infect, most probably that whole population is going to die and progeny phage will have nothing to further proliferate. So it is more wise to be latent and wait for the bacteria to replenish in such cases. On the other way around, having a lot of bacteria to infect around would mean that it is better to go after a lytic cycle.
So how does the phage decide? The decision to undergo lytic or lysogenic cycle depends on certain molecular switches. There has been substantial enquiry into these pathways and there is a reasonable understanding of what is probably happening. However, phages by themselves communicating is not known, untill now. In a recent publication in nature, a team has for the first time, identified a peptide molecule (called as arbitrium) to communicate with one another that literally decides the life cycle choice.
The researchers started with a hypothesis that bacteria secrete communication molecules to alert other bacteria of phage infection. For this, Bacillus subtilis strain 168 were infected by four different phages- phi29, phi105, rho14 and phi3T. A chance observation led to the hypothesis that a small molecule is released to the medium during infection of B subtilis by phi3T (Phenomenon was not observed in other phages) and it probably modulates infection dynmaics of the phage. Further study showed it was a short peptide. The researchers went ahead and sequenced the phi3T genome and made came up with a couple of candidate genes. Of these, one gene exhibited features reminiscent of Bacillus quorum sensing peptides which encoded a short ORF which could be processed by B subtilis extracellular proteases (based on consensus cleavage site for peptide maturation). The predicted peptide was Ser-Ala-Ile-Arg-Gly-Ala (SAIRGA) which was subsequently confirmed by mass spec analysis. The peptide was further verified by showing that there was a dose dependent effect of elevated lysogeny in the presence of the SAIRGA peptide. The gene was named as aimP, which is located just next to another gene that codes for tetratricopeptide repeat (TPR) domain, typical of intracellular peptide receptors of the RRNPP family in quorum sensing systems. This gene turned out to be the receptor. The rest of the paper deals with experiments to identify how this system works.
Transcriptome analysis of phage for effect of arbitrium had a transcript (aimX), that was immediately downstream to the AimR DNA-binding site. This transcript showed substantial expression in the absence of the arbitrium but was severely reduced in presence of the peptide. Based on some more genetic knockdowns and other assays the researchers came up with a model shown in Fig 1.
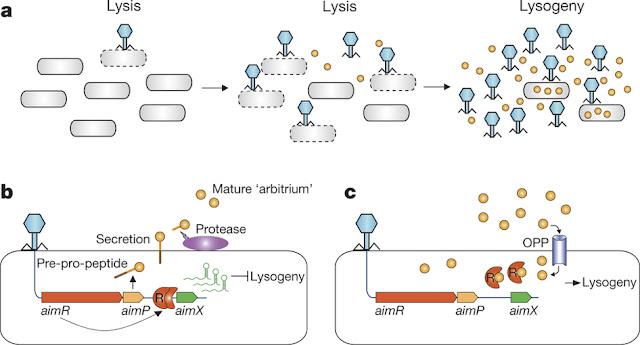 |
| Fig 1: a, Dynamics of arbitrium accumulation during infection of a bacterial culture by phage. b, At the first encounter of a phage with a bacterial population, the early genes aimR and aimP are expressed immediately upon infection. AimR, as a dimer, activates AimX expression. AimX is an inhibitor of lysogeny, possibly as a regulatory non-coding RNA, directing the phage to a lytic cycle. At the same time AimP is expressed, secreted and processed extracellularly to produce the mature peptide. c, At later stages of the infection dynamics, the arbitrium peptide accumulates in the medium and is internalized into the bacteria by the OPP transporter. Now, when the phage infects the bacterium, the expressed AimR receptor binds the arbitrium molecules and cannot activate the expression of AimX, leading to lysogeny preference. Copied from Source |
Earlier, Clokie etal;2014 had published a paper indicating that bacterial quorum sensing systems can be found on phage genomes. Clokie comments on the paper, "I’ve thought about doing those experiments to see if there’s something in the media. Phages broadcast in different frequencies. They speak in different languages and they can hear only the language that they speak". Source
References
Callaway, Ewen. "Do You Speak Virus? Phages Caught Sending Chemical Messages". Nature (2017): doi:10.1038/nature.2017.21313
Erez, Zohar et al. "Communication Between Viruses Guides Lysis–Lysogeny Decisions". Nature 541.7638 (2017): 488-493.
Hargreaves, Katherine R., Andrew M. Kropinski, and Martha R. J. Clokie. "What Does The Talking?: Quorum Sensing Signalling Genes Discovered In A Bacteriophage Genome". PLoS ONE 9.1 (2014): e85131.




Comments
Post a Comment