VPM1002 TB vaccine to be tested in India
Tuberculosis continues to be a high burden problem in many parts of the world especially, its co-infection with HIV creates substantial complications. Though BCG is universally administered, BCG fails to protect after a certain number of years. Research is currently focussed on inventing a totally new vaccine or to create modifications in BCG allowing better vaccine performance. In India, a recombinant vaccine called VPM1002 is planned to be tested for phase II/III vaccine trial. Here are some details of this vaccine.
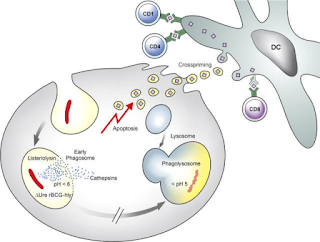 |
Fig 1: Schematic description of the underlying
mechanism of improved T cell stimulation
by VPM1002. Source
|
BCG is derived from M bovis and has immunological properties which is much similar to M tuberculosis. The bacteria is phagocytosed by host macrophages, and are efficiently trapped in cellular phagosome. However, the cell fails to digest the bacteria due to multiple virulence mechanism of the bacteria. One of them is a urease which plays a role in pH neutralization of phagosome thus denying its maturation. Subsequently, antigens are processed by MHC II pathway and induce CD4+ T-cell responses but what is actually required is a CD8+T cell response. In 2005 a JCI paper reported a rBCG (Recombinant BCG) prague strain that secretes listeriolysin of Listeria monocytogenes and is urease C-deficient. The idea being that the a urease deficient mycobacterium secreting listeriolysin allows better phagosome maturation and potentially activating MHC I pathway thus eliciting a desired CD8 response. Vaccine developed from this strain is called as VPM1002. Prof. Kaufmann comments “The vaccine being tested is intended to replace the current BCG vaccine and will be administered to young children to protect them against tuberculosis. Adults may also be able to benefit from it later”. In a phase I Clinical trial conducted in Germany and and South Africa, volunteers were followed up for 6 months after a single vaccination with 5 x 10 5 CFU was safe, well tolerated and induced multifunctional CD4+ and CD8+ T-cells. Studies have also showed that rBCG is significantly better in terms of immunity and safety. The findings were subsequently confirmed by a phase 2a study in South Africa.
 |
| Photo: VPM1002 Vaccine. Source |
India being a high burden for TB desperately needs a new and better vaccine. Serum Institute of India has apparently obtained approval to go ahead with Phase II/III vaccine testing. This will be a double blind placebo-controlled, randomised clinical trial which will see enrolment of 2,000 adults (1000 participants will receive vaccine and the other 1000 will receive placebo) in 17 centres. A single dose will be administered and followed up for 12 months. The work is planned in 2 phases. In the 1st phase, 200 participants will be given the vaccine and safety evaluated. In the second phase (given the success of first phase), the remaining volunteers will be vaccinated. Dr. Prasad S. Kulkarni, Medical Director at Serum Institute says, “Adults who have completed TB treatment will be first screened and enrolled if found eligible 2-4 weeks after completion of TB treatment. Traces of the drugs may be present in the body for two weeks after completion of the treatment. Since the vaccine contains live, weakened bacteria, the drugs can kill them if given earlier than two weeks after completing the treatment.”
The key importance of VPM1002 in contrast with BCG is superior immunity conferred by CD8+ T cells, enhanced Il-17 secretion. It also reduces the risk associated with BCG complications seen in a small subgroup of HIV positive subjects.
References:
Grode L. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. Journal of Clinical Investigation. 2005;115(9):2472-2479.
Grode L, Ganoza C, Brohm C, Weiner J, Eisele B, Kaufmann S. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. 2013;31(9):1340-1348.
VPM Study group. HIV-unexposed newborn infants in South Africa in HIV-unexposed newborn infants in South Africa. Clin. Vaccine Immunol. doi:10.1128/CVI.00439-16




Comments
Post a Comment