Critical analysis of HIV vaccines- Part I
Greetings
STEP trial (Phambili Trial):
RV144 (Thai HIV vaccine) trial
If u have been a regular follower of my blog posts, more recently I have been posting often about vaccines, new vaccine strategies and topics revolving around that. When it comes to the vaccines, there is nothing of a more formidable challenger than the one compared to HIV vaccine. Millions of dollars spent, Years of research knowledge, and where do we stand? Thats a pretty good question to ask and is the topic of this post. When HIV was first discovered, Margaret Heckler declared "We hope to have such a vaccine ready for testing in approximately two years…yet another terrible disease is about to yield to patience, persistence and outright genius". That was in 1984. Even if I say this now, in 2013, I probably am going to be invariably wrong.
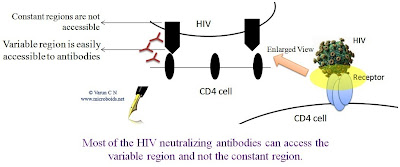 |
| Fig 1: Neutralizing Antibodies cannot access the constant region |
What makes the virus hard to be targeted by a vaccine? There are a couple of reason that can be suggested straight away. First, the virus is nastily variable at regions where antibody can reach. This is attributed a very sloppy reverse transcriptase, that induces a huge lot of variation. If all the different HIV from a single patient could be sequenced, we will note that there is still a plethora of variation. This makes it hard for the immune system to recognize and attack. The 2nd important problem is that the virus attacks the immune system itself- the CD4+ T cells that actually is the master mind of Immunological attack.
One way to get around the problem of attacking the broad range of antigenic configuration is to take the path of Broadly neutralizing antibodies. (I have detailed the theory here). Any given immune response can be considered under 2 headings- Sterilizing immunity (Total wipe out of pathogen) or Non sterilizing immunity (Keeps the pathogen under surveillance and controls pathogen load). What is desirable is sterilizing immunity which requires Neutralization antibodies. However, if that isn't achievable we could still be contend with the other mode.
There have been an zillion candidates tried against HIV, of which 4 have made it to Phase III trials- AIDSVAX (2 versions), STEP trial and RV144 trial. VOICE (Vaginal and Oral Interventions to Control the Epidemic) Trial and CAPRISA 0004 (Centre for the AIDS Programme of Research in South Africa), are other important Clinical studies when it comes to HIV prevention. But they are microbicides based approach, a topic to be talked about separately.
AIDSVAX:
This is a bivalent vaccine consisting of a preparation of recombinant gp120 from two types of HIV, is being developed by VaxGen for the potential prevention of HIV infection. Its earlier version was a monvalent vaccine. The vaccine was reportedly a failure, as per results published in 2003 (multiple papers). The results of AIDSVAX Trial shown below is least impressive. Developments are on way
 |
| Fig 2: AIDSVAX Trial results. Source |
STEP trial (Phambili Trial):
 |
| Fig 3: Anti–HIV-1 T cell responses Source |
The trial was based on the initial findings of MERCK laboratory that concentrated on the cellular arm of immunity. The rationale was to look for candidates that elicit higher CMI. Based on sampling from multiple clades (HIV-1 clades A, B, and C), each were compared by standardized interferon- gamma enzyme-linked immunospot assays among unvaccinated individuals, infected with diverse HIV-1 clades, from Brazil, Malawi, South Africa, Thailand, and the United States. As seen in the Fig 3, though there is a significant difference in immune response, Gag, Pol, Nef, and Env proteins are shown to be best targeted.
 |
| Fig 4: AD5 vaccine, developed by MERCK and NIH Source |
Based on the findings said above, Merck developed the experimental vaccine called V520 to stimulate HIV-specific CMI. The vaccine was made from a replication incompetent Adenovirus vector 5 (Adv5) expressing Gag, Pol, Nef. The STEP trial was actually conducted in US and other countries and the Phambili trial was conducted in South Africa. However, now all the STEP trial is represented as the Phambili trial. Whatever is the case, the trial was halted owing to the observation that the vaccine group presented with higher infection rate than the placebo. That was a hefty un-expected blow. In article that appeared in NEJM (Link), Anthony Fauci, the director of the NIAID was quoted as "“To be brutally honest with ourselves, we have to leave open the possibility. . . that we might not ever get a vaccine for HIV. People are afraid to say that because they think it would then indicate that maybe we are giving up. We are not giving up. We are going to push this agenda as aggressively and energetically as we always have. But there is a possibility— a clear finite possibility — that that’s the case.”
Natural scientific instinct was to look for cause. There were several proposed explanations as to what may be the reason for vaccine failure. The most noted explanation is as follows. In ana experiment where antibodies to adenovirus 5 was combined with the vaccine backbone (Ad5 Immune Complex ), they triggered more notable DC maturation, which increased CD86 expression, decreased endocytosis, and production of tumor necrosis factor and type I interferons. This led to activation of T cells, which are targeted by HIV in the body. In cell culture, T cells succumbed to HIV-1 infection three times more quickly than did those in a mixture that lacked adenovirus 5 antibodies. Since Ad5 is a very common virus and people may easily have antibodies this is a very plausible explanation. There are debates as to if this really is a case, but otherwise is accepted at least as a partial theory.
That leads me to a question. If the Ad5 in itself is the problem, how about an alternative vector. The question seems to be very compelling, but answer is not easy to come. There are alternative adenovirus vectors under development such as Ad35 and Ad41. However, their toxicity and expression profiles are not much different. Another development is the use of modified recombinant Ad5 vector deleted for E1 and E2b deleted. In a study by Osada etal, it was shown that [E1-, E2b-]vectors retaining the Ad5 serotype are potent immunogens in pre-clinical models despite the presence of significant Ad5-specific immunity, in contrast to [E1-] vectors which was used earlier. Other vectors have been explored such as MVA and NYVAC, which are efficient in inducing both cellular and humoral responses against HIV-1.
 |
| Fig 5: Different HIV-1 vaccine strategies Source |
I want to make a digression here. MERCK Ad5 vaccine was not the only vaccine that MERCK has tried or is under process. Other methods used to elicit CMI include- Plasmid DNA CRL 1005 formulation, Pox virus vector based vaccines (Vaccinia, Modified vaccinia Ankara virus; MVA, Canary pox; ALVAC), BCG vector, Alphavirus vector (VEE), Virus Like particles (VLP) and and liposome delivery methods. Of these Plasmid DNA CRL 1005 formulation, Alvac are the ones under active investigation.
RV144 (Thai HIV vaccine) trial
 |
| Fig 6: Efficacy of RV144, microbicides and PrEP Source |
Alvac and AIDSVAX given in combination has been tried in this trial. This involved 4 priming injections of a recombinant canarypox vector vaccine (ALVAC-HIV [vCP1521]) plus two booster injections of a recombinant glycoprotein 120 subunit vaccine (AIDSVAX B/E). The maximum vaccine efficacy was approx about 30%. (Phew!!! After 6 total doses, just 30%?). Experts have argued on both grounds. One group says 30% is not good enough. There are other people who comment, 30% is a good starting point, given the fact that the most promising STEP trial showed negative efficacy. Often the outcome of this trial is compared with the microbicides, as shown in Fig 6.
I will continue regarding our current understanding and breakthroughs in this field, in the next post.
Coplan PM, Gupta SB, Dubey SA, Pitisuttithum P, Nikas A, Mbewe B, Vardas E, Schechter M, Kallas EG, Freed DC, Fu TM, Mast CT, Puthavathana P, Kublin J, Brown Collins K, Chisi J, Pendame R, Thaler SJ, Gray G, Mcintyre J, Straus WL, Condra JH, Mehrotra DV, Guess HA, Emini EA, & Shiver JW (2005). Cross-reactivity of anti-HIV-1 T cell immune responses among the major HIV-1 clades in HIV-1-positive individuals from 4 continents. The Journal of infectious diseases, 191 (9), 1427-34 PMID: 15809900
Steinbrook R (2007). One step forward, two steps back--will there ever be an AIDS vaccine? The New England journal of medicine, 357 (26), 2653-5 PMID: 18160684
Perreau, M. (2008-11-03) Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. , 205(12), 2717-2725. PMID: 18981239
Osada T, Yang XY, Hartman ZC, Glass O, Hodges BL, Niedzwiecki D, Morse MA, Lyerly HK, Amalfitano A, & Clay TM (2009). Optimization of vaccine responses with an E1, E2b and E3-deleted Ad5 vector circumvents pre-existing anti-vector immunity. Cancer gene therapy, 16 (9), 673-82 PMID: 19229288
Osada T, Yang XY, Hartman ZC, Glass O, Hodges BL, Niedzwiecki D, Morse MA, Lyerly HK, Amalfitano A, & Clay TM (2009). Optimization of vaccine responses with an E1, E2b and E3-deleted Ad5 vector circumvents pre-existing anti-vector immunity. Cancer gene therapy, 16 (9), 673-82 PMID: 19229288
Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, & MOPH-TAVEG Investigators (2009). Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine, 361 (23), 2209-20 PMID: 19843557



Pennvax showing promise in early testing.
ReplyDelete