Scientists find a quick method to get Monoclonal Antibodies of interest.
A lot of new emerging infectious diseases are no on global radar and that highlights how unprepared we are in fighting it. Most of these are geographically limited and terminate with a few countable number of cases. The one's like Influenza variants, Zika and Ebola have been more rampant. Though a short term solution is to administer antibiotics or quarantine, the best approach is to vaccinate. The centre of this whole problem lies in B cells.
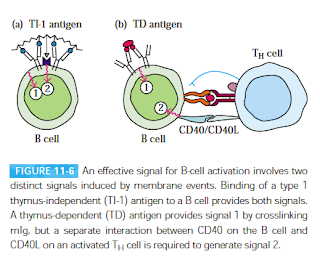 |
| Fig 1: B cell activation. Source: Kuby Textbook; 5e |
I need to visit back the B cell activation pathway. B cells are a type of lymphocytes that is involved in making antibodies. B cells by nature are inactive and have to be activated specifically. The individual lineage of B cells can make antibodies against a specific antigen. These inactive B cells which are competent enough to start making antibodies, provided they have the right signal, is called as immunocompetent B cells.
Depending on the nature of the antigen, there are two modes of B-cell activation. TH cells dependent (TD) and TH cell independent (TI). There are 2 types of signals that are required as membrane events, to activate a B cell. The first activation signal is an antigen binding to B cell receptors (BCRs). Once bound, the antigen is internalized by receptor-mediated endocytosis, digested, and complexed with MHC II molecules on the B cell surface. The second activation signal (also referred as the costimulatory signal) is CD40/CD40L interaction. See Fig 1. Once activated they go on to mature and convert to B cells which start making antibodies. There is some evidence that this is not completely true and there are more signals in the interaction. For example, TLR is possibly the third signal.
There have been several previous attempts in the laboratory to replicate this process in the laboratory condition. Previous studies have shown that patient-derived B cells when treated with CpG oligonucleotides, they stimulate every B cell in the population. CpG oligonucleotides are short single-stranded synthetic DNA molecules that contain a cytosine triphosphate deoxynucleotide followed by a guanine triphosphate deoxynucleotide. The CpG is mainly recognised by TLR 9, which is expressed in B cells and Plasmacytoid dendritic cells and is thus an excellent immunostimulant. Antigen-dependent activation of B cells in-vitro is difficult to achieve result because the wide haplotype variation of MHC IIs necessitates the use of unique T cells specific to a particular MHC II to activate B cells in vitro. This problem was solved by the team led by Facundo Batista, from the Francis Crick Institute in London based on which the current paper is built.
The researchers started with coated streptavidin polystyrene nanoparticles containing a mixture of biotinylated anti-κ antibody and the TLR ligand CpG. The team showed that by treating the patient derived B cells with the coated nanoparticles and the appropriate antigen. In short, CpG oligonucleotides are only internalized into those B cells that recognize the specific antigen coated, and these cells are therefore the only ones in which TLR9 is activated to induce their proliferation and development into antibody-secreting plasma cells.
The team has shown that the results are replicable when done with different bacterial and viral antigens (such as tetanus toxoid and proteins from several strains of influenza A, HIV gp120). The studied showed specifically that in vitro stimulation of memory B cells with particulate antigen-CpG selectively enriched the frequency of CD27hi/CD38hi antigen-specific plasma cells irrespective of the nature of the antigen that was chosen. So technically in proof, this method can be applied to any antigen. Further, it was achieved in a very short time, almost a week.
This novel method is much superior to several other methods such as phage display, EBV immortalization, yeast display, and humanized animal models since it doesn't rely on a large scale screening and identification. Basically, this method allows for selective stimulation of memory B cells from healthy donors, leading to proliferation and differentiation into plasma cells that produce antigen-specific antibodies, even in antigen-naive donors (They demonstrated by showing you could develop antibodies against HIV from cells derived from HIV negative donors), which means making therapeutic vaccines (Antibodies) could be very fast.
As Facundo Batista explains, "Specifically, it should allow the production of these antibodies within a shorter time frame in vitro and without the need for vaccination or blood/serum donation from recently infected or vaccinated individuals. In addition, our method offers the potential to accelerate the development of new vaccines by allowing the efficient evaluation of candidate target antigens."
References:
Irene Sanjuan Nandin, Carol Fong, Cecilia Deantonio, Juan A. Torreno-Pina,Simone Pecetta, Paula Maldonado, Francesca Gasparrini, Jose Ordovas-Montanes, Samuel W. Kazer, Svend Kjaer, Daryl W. Borley, Usha Nair, Julia A. Coleman, Daniel Lingwood, Alex K. Shalek Eric Meffre, Pascal Poignard, Dennis R. Burton, and Facundo D. Batista. Novel in vitro booster vaccination to rapidly generate antigen-specific human monoclonal antibodies. The Journal of Experimental Medicine, 2017 DOI: 10.1084/jem.20170633.
Eckl-Dorna J, Batista F. BCR-mediated uptake of an antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009;113(17):3969-3977.





Comments
Post a Comment