Critical analysis of HIV vaccines- Part II
Greetings
Gag and Env are good immunogens, there maybe a good way to target them. Studies showing correlation between the antibodies and viral loads have been published. What comes as a surprise is that Gag antibody levels are correlated with reduced viral load, and Env-Ab levels with Increased viral load. I have not found an explanation for this phenomenon. The study done in a small population, has brought in the question of is Env antigen a candidate for vaccine? Moreover, studies have shown that there is a strong association between gag and env evolution, and env sequence diversion is higher in chronic patients. Of course, every single variation will not make an appearance, for obvious reasons and a natural molecular selection plays significant role. However, even a 1% increase in divergence is significantly linked to higher viral load, as the immune system has to re calibrate the response.
Yesterday, I had posted about the basic facts of what makes an HIV vaccine difficult, The AIDSVAX and STEP trial which failed. I also talked about the possible reasons of why the vaccine probably failed, and what research is on way to overcome the problem. Continuing from my earlier post, here I talk about alternative options under development that may interest the researchers.
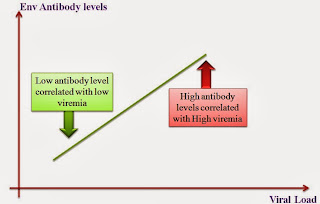 |
| Fig 1: Env Ab negative correlation with HIV viremia |
 |
Photo 1: Intradermal Electroporation
Applicator. Source
|
Am highly tempted to argue that possibly Gag represents a good candidate, atleast one which can direct useful antibodies. A very recent publication uses a DNA vaccine approach. Programmed death-1 (PD1) linked to HIV-1 GAG p24 antigen (Fusion DNA product), delivered by intramuscular immunization via electroporation (EP), in mice elicited consistently high frequencies of GAG-specific, broadly reactive, polyfunctional, long-lived, and cytotoxic CD8+ T cells and robust anti-GAG antibody titers. Though Electroporation is not a good option for human use, this maybe tweaked using vector delivery system, and hence is a possible approach. A yet another study by Inovio Pharmaceuticals (Link), where similar approach was conducted using DNA plasmids targeting the gag, pol, and env proteins of HIV-1, delivered by electroporation (CELLECTRA® electroporation device) . In a announcement reported on Mar 13, 2012, the study claimed that the study had achieved a strong T cell immune responses in a Phase I clinical study targeting treatment of the HIV subtype prevalent in North America and Europe, in HIV-positive subjects.
In my previous post, I had said that RV144 had achieved an efficacy of about 30% and represented an important breakthrough. The study has been further advanced as a follow up known as RV305. The study was conducted by MHRP (U.S. Military HIV Research Program) in Thailand to evaluate re-boosting in volunteers who participated in the RV144 study. The next step- RV306 was expected to be launched as soon as posible. It will be aimed at comparing additional vaccine boosts in 360 new volunteers. RV306 study will be conducted at three sites: the Vaccine Trial Centre at Mahidol University and the Royal Thai Army Armed Forces Research Institute of Medical Sciences (AFRIMS), both in Bangkok, and the Royal Institute for Health Sciences (RIHES) in Chiang Mai. Lisa Reilly, the MHRP’s communications director says, "It is not an efficacy study, so it does not need to be large… We are hoping to conduct an efficacy study with an improved vaccine boost/adjuvant in Thailand, but it will not start until 2016/17". Source
That brings me to the last but the latest research in HIV vaccination. What is probably the most important factor that hampers the HIV vaccine efficacy? There are once again several set of answers in this area. Other than the fact that virus keep changing for immune system to keep track (Already discussed in previous post), HIV-1 cannot replicate in dendritic cells (DCs), and that HIV-1 replication in macrophages is not very efficient. This is sometime referred as lack of immune stimulation. HIV-2 can replicate well in these cells (Posses Vpx, which counteract inhibitory effect of SAMHD1 in these cells). Point is they are less pathogenic. So one of the hypothesis was if we could the immune system constantly could be surveilling for HIV maybe we can achieve non sterilizing immunity.
Following up on the hypothesis, Picker etal, constructed a RhCMV ( rhesus cytomegalovirus vector), encoding the simian immunodeficiency virus (SIV) mac239 proteins Env, Pol, Gag, and Vpr/Vpx. This established persistent, high frequency, SIV-specific effector-memory T cell (TEM) responses at potential sites of SIV replication in rhesus macaques (RM) and stringently control highly pathogenic SIVmac239 infection early after mucosal challenge. The study compared 4 groups-
*Group A- 12 macaques were given the rhCMV/SIV viral vector-based vaccine
*Group B- 12 received an rhCMV/SIV vector-based candidate followed by a replication-defective adenovirus serotype 5 (Ad5) vector-based candidate encoding the full SIVmac239 genome
*Group C- 9 received a DNA prime/Ad5 boost
*Group D- 28 unvaccinated control animals
In this setup, the Group C and D, exhibited typical progressive SIV infection. The Group A and B showed complete control of SIV. This study land- marked a proof of concept. In a recent follow up study published by the same group, showed that the vaccine suppresses SIV to undetectable levels in about 50% of the animals after vaginal and intravenous challenge as well. U can listen to the authors views on this study here.
I want to call your attention to the STEP trial failure. The vaccine was awesomely successful in primate models. The failure in clinical trial has been attributed to Ad5 antibodies. Given the fact that CMV is also a common infection of humans, antibodies will be present against CMV. Will this cause the same effect as of STEP trial?? I couldn't find an answer to this case. Perhaps, I need to wait for some more data or I haven't grabbed the concept well.
But one comment for sure. We have a long way to go
I want to call your attention to the STEP trial failure. The vaccine was awesomely successful in primate models. The failure in clinical trial has been attributed to Ad5 antibodies. Given the fact that CMV is also a common infection of humans, antibodies will be present against CMV. Will this cause the same effect as of STEP trial?? I couldn't find an answer to this case. Perhaps, I need to wait for some more data or I haven't grabbed the concept well.
But one comment for sure. We have a long way to go
Piantadosi A, Chohan B, Panteleeff D, Baeten JM, Mandaliya K, Ndinya-Achola JO, & Overbaugh J (2009). HIV-1 evolution in gag and env is highly correlated but exhibits different relationships with viral load and the immune response. AIDS (London, England), 23 (5), 579-87 PMID: 19516110
Zhou J, Cheung AK, Tan Z, Wang H, Yu W, Du Y, Kang Y, Lu X, Liu L, Yuen KY, & Chen Z (2013). PD1-based DNA vaccine amplifies HIV-1 GAG-specific CD8+ T cells in mice. The Journal of clinical investigation, 123 (6), 2629-42 PMID: 23635778
Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M Jr, Lifson JD, & Picker LJ (2011). Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature, 473 (7348), 523-7 PMID: 21562493
Hansen SG, Jr MP, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Früh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, & Picker LJ (2013). Immune clearance of highly pathogenic SIV infection. Nature PMID: 24025770
Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M Jr, Lifson JD, & Picker LJ (2011). Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature, 473 (7348), 523-7 PMID: 21562493
Hansen SG, Jr MP, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Früh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, & Picker LJ (2013). Immune clearance of highly pathogenic SIV infection. Nature PMID: 24025770





Comments
Post a Comment