No Tit for HIV TAT
Greetings
In my previous blog post, I had put up our current understanding of HIV latency. Of course that was a very short post, and by no means, represent the complete picture of what we know. But, it did spill the idea in the right place. As I have pointed out in my previous post, TAT (Trans-Activator of Transcription), is essentially involved in regulating the latency and HIV genome expression. Its a common knowledge that HIV TAT is expressed in high quantities in circulation. It is almost evident from current research that the TAT contributes to additional pathology of HIV, especially in Neuro-AIDS. That is interesting enough to be worth a discussion here.
HIV TAT is a central molecule of HIV (If I may say so). Once a proviral DNA is integrated into the host genome, the expression initially is controlled by a promoter sequence that can be activated by NF-kB. However, this usually is not enough, as it doesn't produce complete RNA (Owing to multiple blocks). Short transcripts produced during the course, allows making of TAT, that along with TAR (trans-activating response element), removes the block thereby allowing complete transcription. This forms the central cellular circuit in deciding HIV genetic expression.
The second role of HIV TAT is to help in increasing HIV infection itself. How? Studies indicate that the HIV TAT is secreted in detectable quantities. The TAT uptake is by receptor mediated endocytosis using heparan sulfate (HS) proteoglycans, CD26 or LDL (low-density lipoprotein) receptor - related proteins. The TAT subsequently induces transcription which leads to production of IL-2, IL-6 Tumor necrosis factor, CXCR4 and CCR5 cytokine co-receptors, Angiongenic factors etc. This increases inflammation, and simultaneously brings in new susceptible cells to the scene. Perhaps of all the effects that in extracellular environment, the most important is its role in Neuro-AIDS (Especially its possible roles in AIDS dementia complex (ADC).
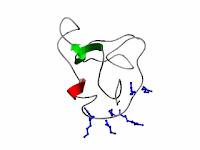 |
| Fig 1: Structure of HIV TAT Source |
 |
| Fig 2: TAT toxicity |
 |
| Fig 3: Effects of TAT |
The mechanism of neurotoxicity of TAT is not well understood, is a subject of intense research in the field of Neurovirology. The current idea is that TAT effects, Neurons, astrocytes and Monocytes in CNS leading to extensive damage. Studies on human fetal neurons with TAT showed that the damage is independent of Na+ and preliminary studies suggested that TAT is possibly directly involved. The most widely accepted mode of action is speculated to be through production of reactive NO species (See Fig 4). TAT is also know to activate apoptosis in neuronal cells via activation of glutamate receptors of the non-N-methyl-D-aspartate. In the case of astrocytes, the same NO based damage can be initiated via interaction through CCL2 receptors. TAT can also directly up- regulate TNF-α dependent MCP-1/CCL2 production via MEK/ERK pathway. As mentioned earlier, the Endosome based uptake is the chief mechanism of TAT uptake in cells. Hence it is reasonable to find that the TAT toxicity is also mediated through interfering with the endolysosomal functioning. This property has been attributed to arginine-rich domain of HIV-1 Tat between amino acid residues 49 and 57.
Safe to say, we don't have a good theraupeutic against HIV TAT, which can significantly help the HIV patients.
 Tyagi M, Rusnati M, Presta M, & Giacca M (2001). Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. The Journal of biological chemistry, 276 (5), 3254-61 PMID: 11024024
Tyagi M, Rusnati M, Presta M, & Giacca M (2001). Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. The Journal of biological chemistry, 276 (5), 3254-61 PMID: 11024024
 |
| Fig 4: TAT based Neurotoxicity |
The above will leave an impression that TAT is a highly potential candidate to be attacked. There are 2 approaches that we can think of here. The first ofcourse is a vaccine design the other being pharmacological intervention. TAT antibodies are demonstrable in populations infected with HIV. The antibody response has been demonstrated in the vaccines- (i) TAT dominant B-cell epitope vaccine, using CyaA vector, which targets dendritic cells (ii) TUTI-16 is a synthetic universal HIV-1 Tat epitope vaccine. TAT inhibitors such as didehydro- Cortistatin A; dCA (derivative of Cortistatin A) has been shown to have significant activity. Of these, however to date there is no clinically approved therapeutic.
Safe to say, we don't have a good theraupeutic against HIV TAT, which can significantly help the HIV patients.
Hazleton JE, Berman JW, & Eugenin EA (2012). Purinergic receptors are required for HIV-1 infection of primary human macrophages. Journal of immunology (Baltimore, Md. : 1950), 188 (9), 4488-95 PMID: 22450808
Séror C, Melki MT, Subra F, Raza SQ, Bras M, Saïdi H, Nardacci R, Voisin L, Paoletti A, Law F, Martins I, Amendola A, Abdul-Sater AA, Ciccosanti F, Delelis O, Niedergang F, Thierry S, Said-Sadier N, Lamaze C, Métivier D, Estaquier J, Fimia GM, Falasca L, Casetti R, Modjtahedi N, Kanellopoulos J, Mouscadet JF, Ojcius DM, Piacentini M, Gougeon ML, Kroemer G, & Perfettini JL (2011). Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. The Journal of experimental medicine, 208 (9), 1823-34 PMID: 21859844
King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006 Apr; 8(5): 1347-57. PMID: 16697675
Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, & Nath A (1995). Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Annals of neurology, 37 (3), 373-80. PMID: 7695237
Hui L, Chen X, Haughey NJ, & Geiger JD (2012). Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN neuro, 4 (4), 243-52 PMID: 22591512
Fayolle C, Davi M, Dong H, Ritzel D, Le Page A, Knipping F, Majlessi L, Ladant D, & Leclerc C (2010). Induction of anti-Tat neutralizing antibodies by the CyaA vector targeting dendritic cells: influence of the insertion site and of the delivery of multicopies of the dominant Tat B-cell epitope. Vaccine, 28 (42), 6930-41. PMID: 20728521
D'Orso I, Grunwell JR, Nakamura RL, Das C, & Frankel AD (2008). Targeting tat inhibitors in the assembly of human immunodeficiency virus type 1 transcription complexes. Journal of virology, 82 (19), 9492-504. PMID: 18667497
Guillaume Mousseau etal. Potent suppression of HIV viral replication by a novel inhibitor of Tat. Retrovirology. 2012; 9 (Suppl 1): O11. PMCID: PMC3360218





Comments
Post a Comment