SER109- Phase 2 trial doesn't look good
Greetings
A really long time back, I had written about FMT (Fecal Microbiota Transplant). There have been a large number of studies for more than 2-3 years showing that FMT is beneficial and restoring a healthy microbiome is the way to look forward to conditions related to the gut. It has also been predicted that if we could make a synthetic microbiome of right combination, that would be helpful to people such as one's who are on antibiotics for a long time.
There are a lot of reasons to why FMT in its current raw form is not ideal. The most challenging reason is the requirement for donor screening and potential ability to transmit infections. Since there is no practical test that can screen for every possible pathogen, there is always a given risk in FMT directly from a donor. An idealistic approach would be to synthetically make the right combination in the pharmacy and give it as a pill. The problem is we really have no idea about what is the right combination.
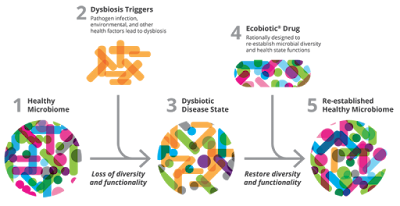 |
| Fig 1: Concept of Ecobiotic drugs. Source |
In a study sponsored by Seres Therapeutics for recurrent CDI (Clostridium difficile infection) efficacy of SER-109 was evaluated. SER-109 was prepared from seven adult donors of stool specimens which were collected and treated with Ethanol to remove vegetative forms, spores washed and used. The preparation mainly contains about 50 species of Firmicutes spores. A vial administered orally as a single dose contains 1 X 108 bacterial spores. The initial report of the study showed that Per-protocol efficacy was about 86% over 8 weeks of follow-up. The company is also developing a product called SER-262 which targets primary CDI. The formulation contains spores from 12 strains. The company calls this concept of re-establishing a healthy microbiome as Ecobiotics.
On 29th July 2016; Seres announced interim results of Phase 2 trial of 89 people, but results look less promising. The study was conducted at 36 centres across the United States. Quoting from Business Wire Eight-week study data shows that the pill simply failed.
In subjects <65 years old, CDI recurrence occurred in 43% of subjects who received SER-109 (12 of 28) and in 27% of subjects who received placebo (4 of 15). In subjects ≥65 years old, CDI recurrence occurred in 45% of subjects who received SER-109 (14 of 31), and in 80% of those who received placebo (12 of 15).
There are a lot of questions that scientists are asking with this finding. The very first question is what was different between Phase 1 and 2 which explains the failure. I have some questions here but I would want to reserve myself till I see a publication about it with details. I also would like to have a sequencing data of SER-109 and know what exactly is in there.
For the time being this press release shows that we are not yet into microbiome pill, partly because we don't completely understand the details.
Reference
Khanna S, Pardi D, Kelly C, Kraft C, Dhere T, Henn M et al. A Novel Microbiome Therapeutic Increases Gut Microbial Diversity and Prevents Recurrent Clostridium difficile Infection. Journal of Infectious Diseases. 2016;214(2):173-181. Link




.svg.png)
Comments
Post a Comment