Search
Sharing Knowledge improves Knowledge... Knowledge should come at as less cost as possible.
Posts
Showing posts from September, 2013

Posted by
Varun C N
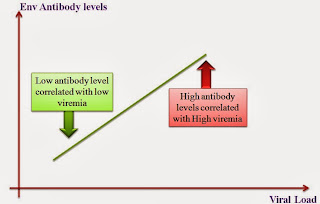
HIV restriction factor- Mx2
- Get link
- X
- Other Apps
Posted by
Varun C N
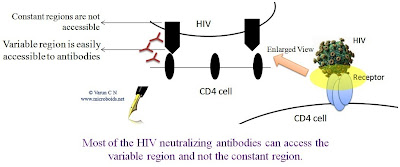
Critical analysis of HIV vaccines- Part II
- Get link
- X
- Other Apps
Posted by
Varun C N
Critical analysis of HIV vaccines- Part I
- Get link
- X
- Other Apps

Posted by
Varun C N
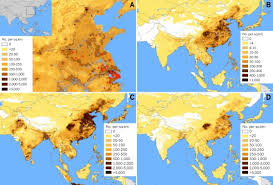
Headlines- MERS and MERS vaccine
- Get link
- X
- Other Apps
Posted by
Varun C N
SAM vaccine for H7N9
- Get link
- X
- Other Apps
Posted by
Varun C N
No Tit for HIV TAT
- Get link
- X
- Other Apps
