Retroviral protection
Greetings
What would be the virus that would be of most interest to you? The answer would be very diverse, depending on what you work and what excites you. But if I had ask you to name a virus taxa that has both undefeated pathogen and something that probably has helped you to live, you will be clearly stumped. One of the very earlier posts of this blog, I talked about retroviruses (Link), and how over the evolution humans owe very existence to retroviruses. Of course I was at that time talking about HERV's or Human endogenous retrovirus. So let us extend that discussion a little further.
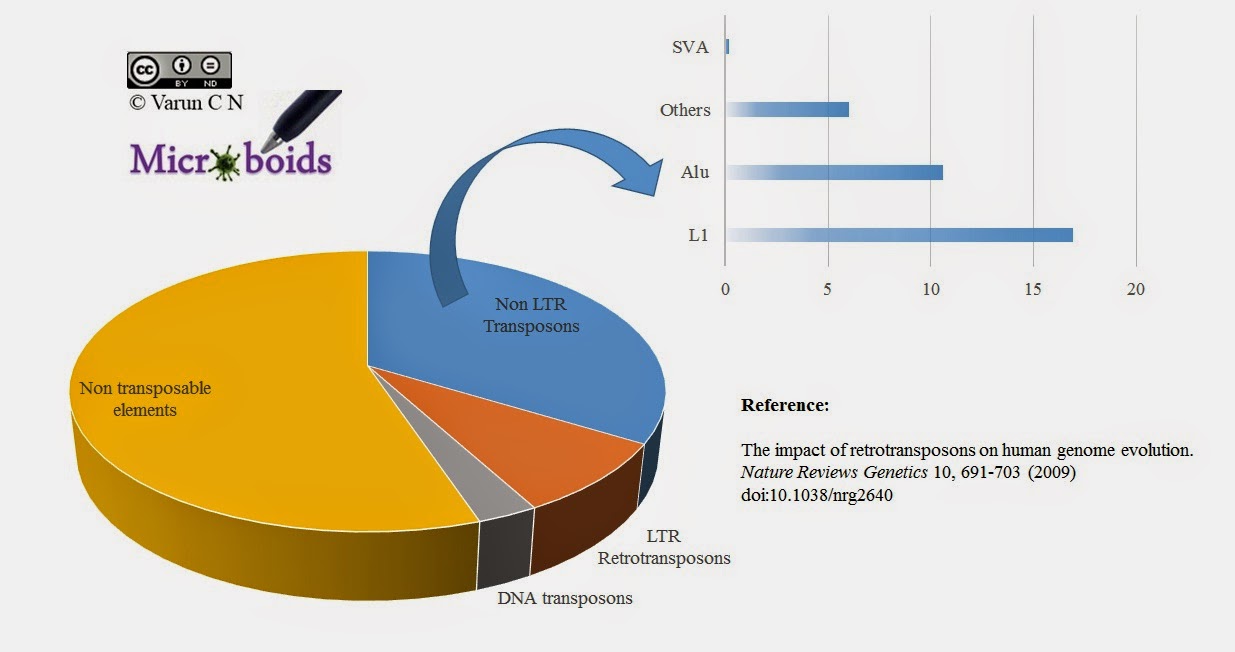 |
| Fig 1: Retroviral elements distribution in Humans. |
I need to digress a little bit here. Though the emphasis is on endogenous retroviral elements, in depth genetic analysis have shown that non retroviral elements such as Bornavirus like elements (Link), Hepatitis C elements, Ebola/Marburg elements (Link) etc in mammalian lineages. But these are random events. It is speculated that these elements have been integrated to genome by active retroviral enzymes that happened to be active when the other virus was in the process. On the other hand retroviral integration are universal in humans. I however am not aware of how widespread is Endogenous Non retroviral elements (ENRE) is in human population.
The role of some retoviral coded proteins such as Syncytin- 1 (coded by HERV-W) and Syncytin-2 (Coded by HERV-FRD) cannot be challenged, and was accepted as a norm. They form the basis of formation of placental membrane. The tide of the story changed with studied showing that placental tissue is highly resistant to infections. An article from Coyne etal showed that cultured primary human placental trophoblasts was highly resistant to infection by a number of viruses in laboratory testing conditions. This was mediated by by exosome-mediated delivery of specific microRNAs. Work by others also showed that HERV's were more active in the placenta and correlated with immunity. This is probably mediated through placental micro vesicles (PMV). PMV are known to circulate in maternal circulation with a role in immunity. Further syncytin 1 is shed from the placenta in association with MV which has direct role. In other words, HERV products are actively involved in placental immunity.
A recent paper in nature, looks a little deep into what the HERV's has to offer for human development. The paper looked into 2 main aspects- When is HERV expression activated and how does it effect immune system. The study implied that HERV-K, which is the most recent member to be integrated into human genome, is activated at the eight-cell stage and continues to do so till the emergence of epiblast cells. Viral like particles (in this case it was essentially gag proteins) was demonstrable in the blastocyst cells.
 |
| Fig 2: HERV expression pattern. Source |
Here's the interesting part. HERV-K is know to produce a protein called as Rec (Analogus to Rev protein in HIV), which is derived through an alternate splicing mechanism from Env gene. Rec is a helper for importing viral products from nucleus to cytoplasm. The authors hypothesized that by bringing the viral materials into the cytoplasm, an innate antiviral defense is triggered. As a proof of this idea an innate antiviral factor IFITM1 mRNA transcription was seen to be increased. IFITM1 (interferon induced transmembrane protein 1), also known as FRAGILIS2 is an IFN-induced antiviral protein which inhibits the entry of viruses to the host cell cytoplasm, permitting endocytosis, but preventing subsequent viral fusion and release of viral contents into the cytosol. Experiments also found that Rec alone was sufficient to account for IFITM1 up-regulation. The relevance of this was tested by challenging against Influenza H1N1 and found that indeed Rec induced IFITM1 was at least partially accounting for resistance.
Earlier paper's have suggested the involvement of miRNA's being effected by HERV activation and subsequent immune modulation. It has also been suggested by some that Endogenous retrovirus can inhibit other competing similar retrovirus from establishing (this phenomenon is known as xenotropism). In this paper innate immune stimulation has produced immunity. Perhaps the net effect is a combination of multiple pathways and effects. some parts HERV genome is still kept active for some reason. As Villarreal puts it, “These viruses have the genetic tools to refashion the hosts' genes, influencing which are active and when, and with which other genes they interact. This means they have the ability to reshape the physical characteristics of their hosts. It's a massive dynamic pool of colonizing genomes."
Now a question arises, as to why is this activity only during embryonic stages. Doesn't it make sense to keep being active so that we can avoid being infected by virus, if these mechanisms are so potent. I don't have an answer for this question. But I know that HERV activity in adults have been correlated with many conditions such as schizophrenia. Probably the HERV mediated immunity being a non specific one is silenced when development reaches a stage where other factors can take over. I think literature is going to pour on this question in coming time.
Cordaux, R., & Batzer, M. (2009). The impact of retrotransposons on human genome evolution Nature Reviews Genetics, 10 (10), 691-703 DOI: 10.1038/nrg2640
Horie M, Honda T, Suzuki Y, Kobayashi Y, Daito T, Oshida T, Ikuta K, Jern P, Gojobori T, Coffin JM, & Tomonaga K (2010). Endogenous non-retroviral RNA virus elements in mammalian genomes. Nature, 463 (7277), 84-7 PMID: 20054395
Douville RN, & Nath A (2014). Human endogenous retroviruses and the nervous system. Handbook of clinical neurology, 123, 465-85 PMID: 25015500
Holder BS, Tower CL, Forbes K, Mulla MJ, Aplin JD, & Abrahams VM (2012). Immune cell activation by trophoblast-derived microvesicles is mediated by syncytin 1. Immunology, 136 (2), 184-91 PMID: 22348442
Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE, Sadovsky Y, & Coyne CB (2013). Human placental trophoblasts confer viral resistance to recipient cells. PNAS, 110 (29), 12048-53 PMID: 23818581
Grow EJ, Flynn RA, Chavez SL, Bayless NL, Wossidlo M, Wesche DJ, Martin L, Ware CB, Blish CA, Chang HY, Reijo Pera RA, & Wysocka J (2015). Intrinsic retroviral reactivation in human preimplantation embryos and pluripotent cells. Nature PMID: 25896322




Comments
Post a Comment